
InhiBET™ BET Inhibitor Platform
VYNE is developing novel therapies for immuno-inflammatory (I&I) diseases that target bromo-domain and extra-terminal domain proteins (BET).
InhiBET™ BET Inhibitor Platform:
BETs are a family of proteins characterized by two bromo-domain subunits (BD1 and BD2) plus an extra-terminal domain (EXT). BET proteins are believed to play a key role in the regulation of inflammatory and oncogenic genes involved in several diseases.
VYNE’s BET inhibitors (BETi’s) are based on its proprietary InhiBETTM platform and small molecule library.
InhiBET™ BET Inhibitor Programs:
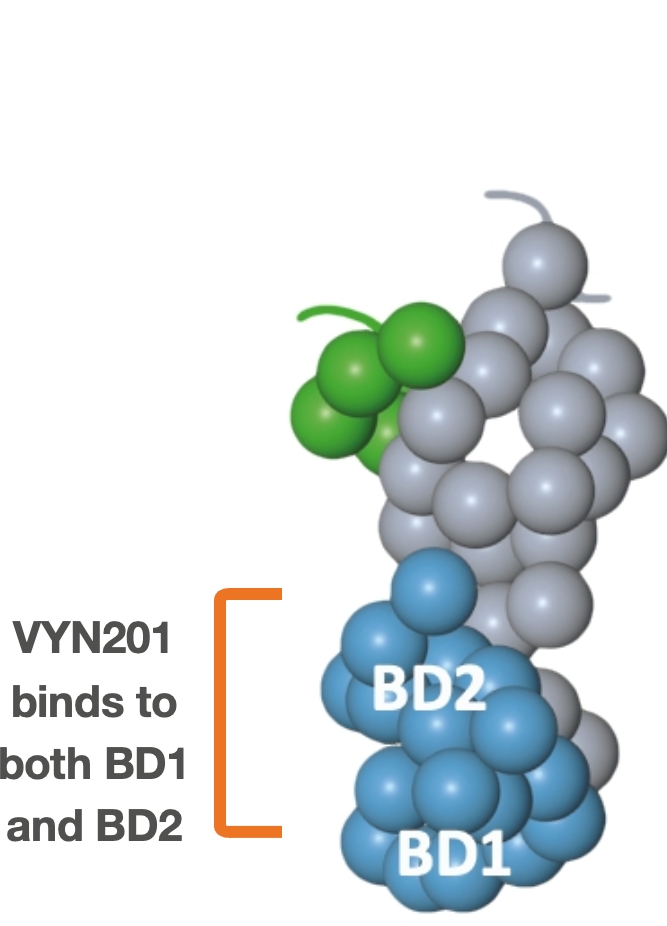
Repibresib (VYN201): Locally administered Pan-BD BET inhibitor
Target Markets:
Designed to address diseases involving multiple, diverse inflammatory cell signaling pathways with low systemic exposure
- Lead indication – Vitiligo
- Other indications benefiting from local application and “soft drug” approach
Broad activity:
- Binds to BD1 and BD2 domains
Status:
- Seeking development partner for program
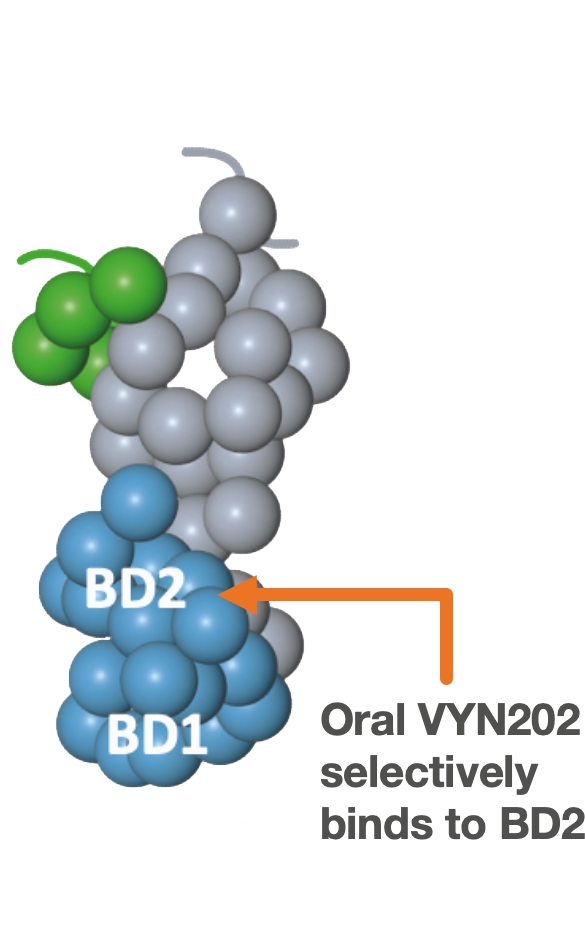
VYN202: Oral BD2-selective BET inhibitor
VYN202 has been designed to achieve class-leading selectivity (BD2 vs. BD1), maximum potency versus BD2 and optimal oral bioavailability. Maximizing on-target potency vs. BD2 and minimizing affinity to BD1 may be the key to optimizing the benefit/risk profile of BET inhibitors for autoimmune diseases.
Target Markets:
- Serious, immune-mediated diseases with limited effective treatment options
Focused activity:
- VYN202 is believed to be the most potent and BD2-selective BET Inhibitor in clinical development1 which is designed to improve efficacy and tolerability
Status:
- Q4 2024: Phase 1a SAD/MAD Complete
- July 2025: Promising preliminary Phase 1b moderate-to-severe PSO data; no further enrollment of study
- Repeat 12-week non-clinical toxicology study ongoing2
1 Based on readily available public information such as clintrials.gov, academic publications and corporate websites/presentations.
2 The repeat non-clinical toxicology study in dogs is intended to remedy the partial clinical hold in male clinical subjects.
